Gynecology Case Studies
Case Study 1: Engineering a Minimally Invasive Solution for Uterine Care
A surgical system designed to remove uterine fibroids, polyps, and/or retained products of conception (RPOC) using a minimally invasive hysteroscopic approach with no incisions.
The procedure is quick and straightforward, allowing most patients to return to regular daily activities within a few days. Hysteroscopic techniques are also the preferred treatment for polyps and fibroids in women of child-bearing age.
Industry
Gynecology
Impact
- Supported our client’s first-in-human studies.
- Scaled production to meet rapidly growing market demand.
- Enabled the launch of a differentiated, single-use hysteroscope in a competitive gynecology market.
Challenge
The client sought to develop a handheld, single-use hysteroscopic system incorporated with fluid management and distention to offer a minimally invasive solution for the removal of uterine fibroids, polyps, and retained products of conception (RPOC). The device had to be intuitive, safe, and suitable for outpatient procedures without requiring incisions or anesthesia and products from other manufacturer.
Solution
As a contract developer and manufacturer, our team partnered with the client from concept through commercialization. We:
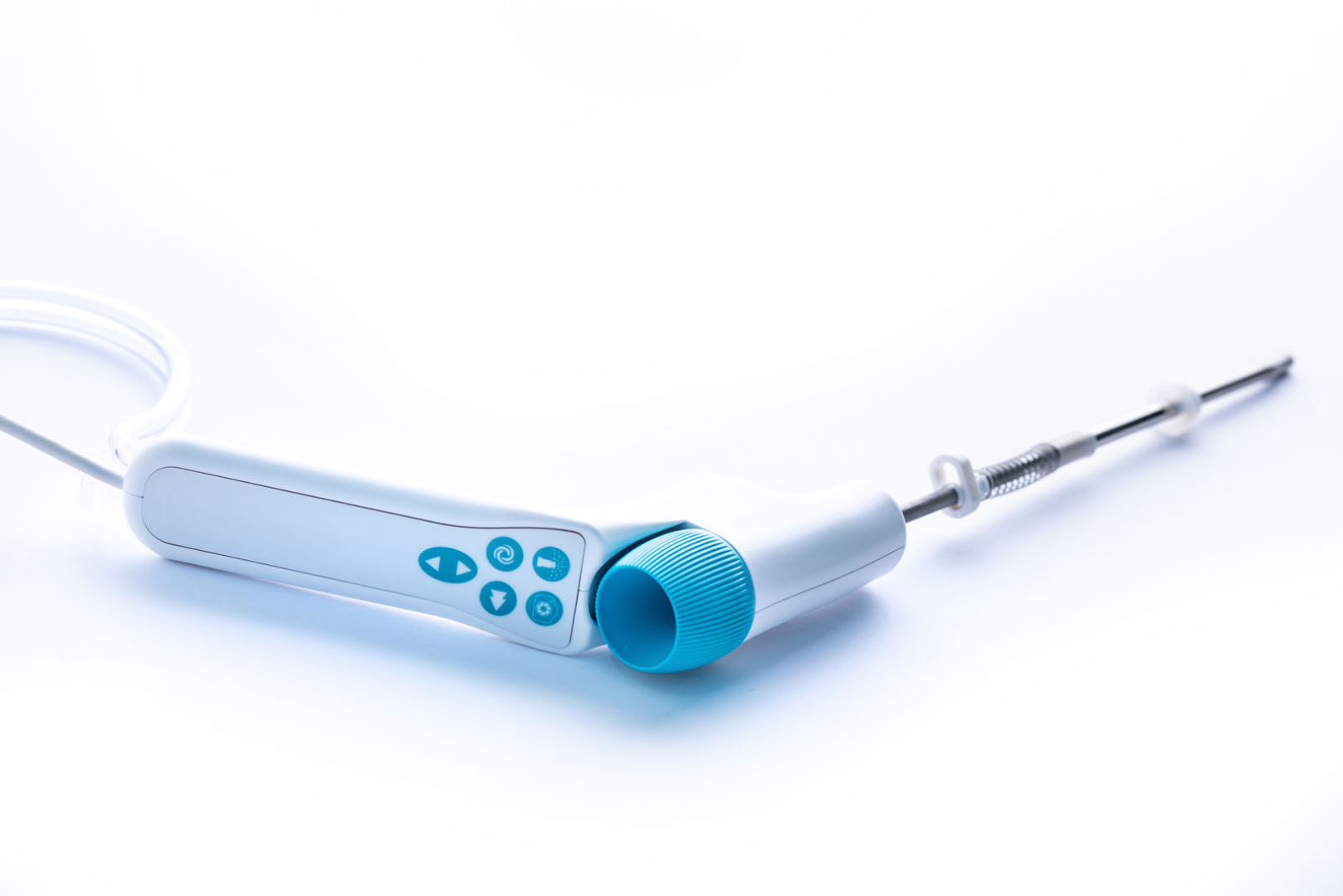
Case Study 2: Advancing Endometrial Ablation Technology
A next-generation surgical system designed for the controlled ablation of the endometrium, the innermost layer of the uterus. The device operates by ionizing argon gas to create plasma within a silicone membrane at the distal tip, which is then used for precise power delivery for tissue ablation. This approach offers a safer and less invasive treatment option, aiming to reduce the need for full hysterectomies.
Industry
Gynecology
Impact
- Supported regulatory submission for FDA approval.
- Accelerated the development process from prototype to commercial production.
- Delivered a safer, minimally invasive treatment alternative for women’s health.
Challenge
The client aimed to develop a surgical system for controlled endometrial ablation that was both effective and less invasive than traditional methods and better outcome for the patients. The device needed to be safe, intuitive, and capable of reducing the number of patients requiring hysterectomy, while meeting strict regulatory requirements.

Solution
As a contract development and manufacturing partner, we:
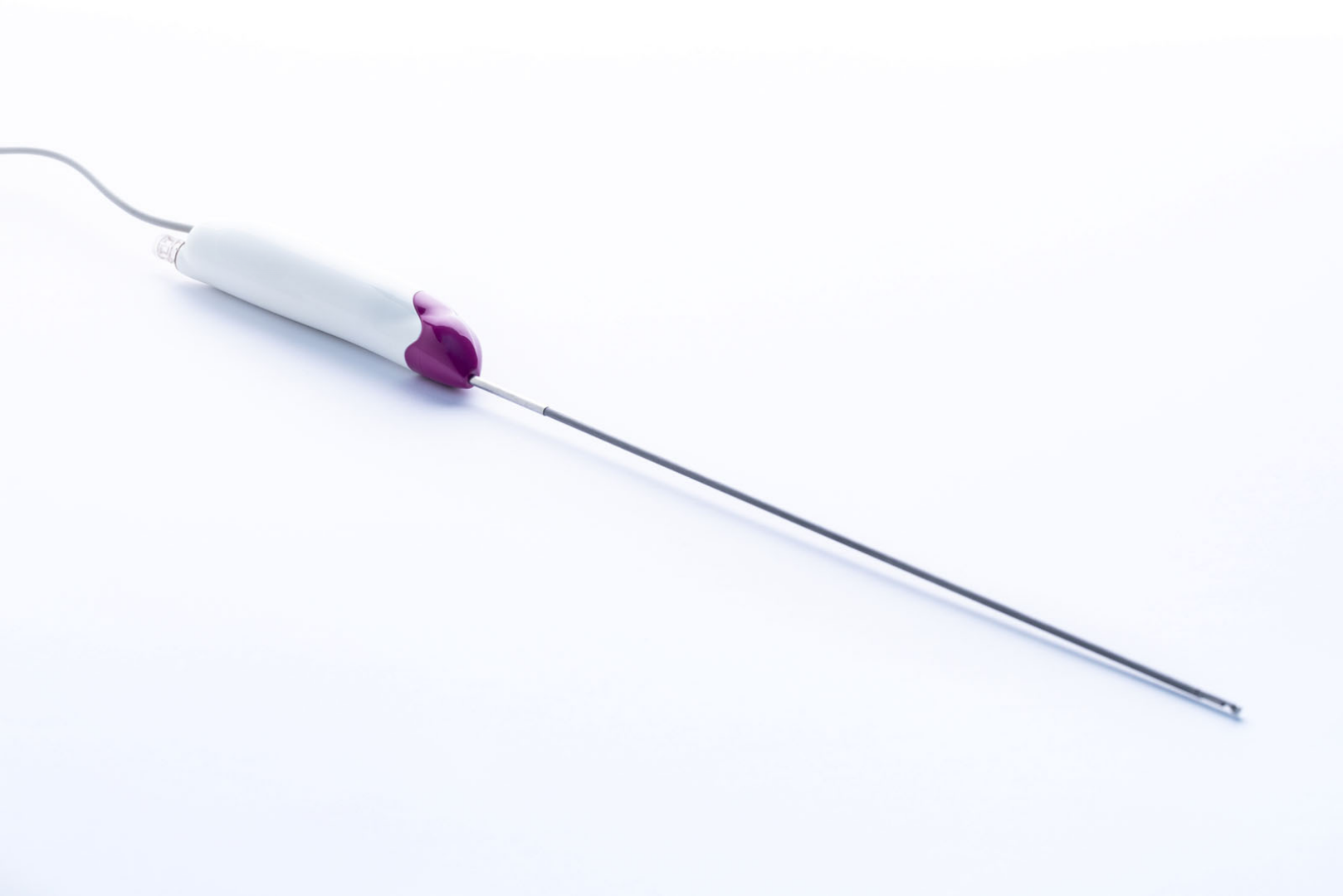
Case Study 3: Innovating Fibroid and Polyp Removal
A next-generation surgical system designed for the hysteroscopic removal of fibroids (myomas) and polyps. The device combines bladeless resection with intrauterine pressure-regulated saline filtration and circulation, offering a safer and less invasive alternative to common surgical approaches, while preserving fertility.
Industry
Gynecology
Impact
- Achieved FDA clearance in less than 24 months from project start.
- Manufactured approximately 1,000 devices for testing and clinical trials.
- Contributed to a successful acquisition by a major medical device company.
Challenge
The client sought to develop a surgical system capable of removing fibroids and polyps in a way that minimized patient risk and preserved reproductive health. The system needed to integrate bladeless resection technology with advanced fluid management, meet strict regulatory requirements, and move quickly from concept to market readiness.
Solution
As a contract development and manufacturing partner, we:
Contact us
Interested in our services? Send us a message and we will get back to you shortly.
Connect with us
Quick Links
By submitting this form, I acknowledge that I have read and understood the Privacy Policy and agree to the processing of my personal data for the purposes stated therein.

