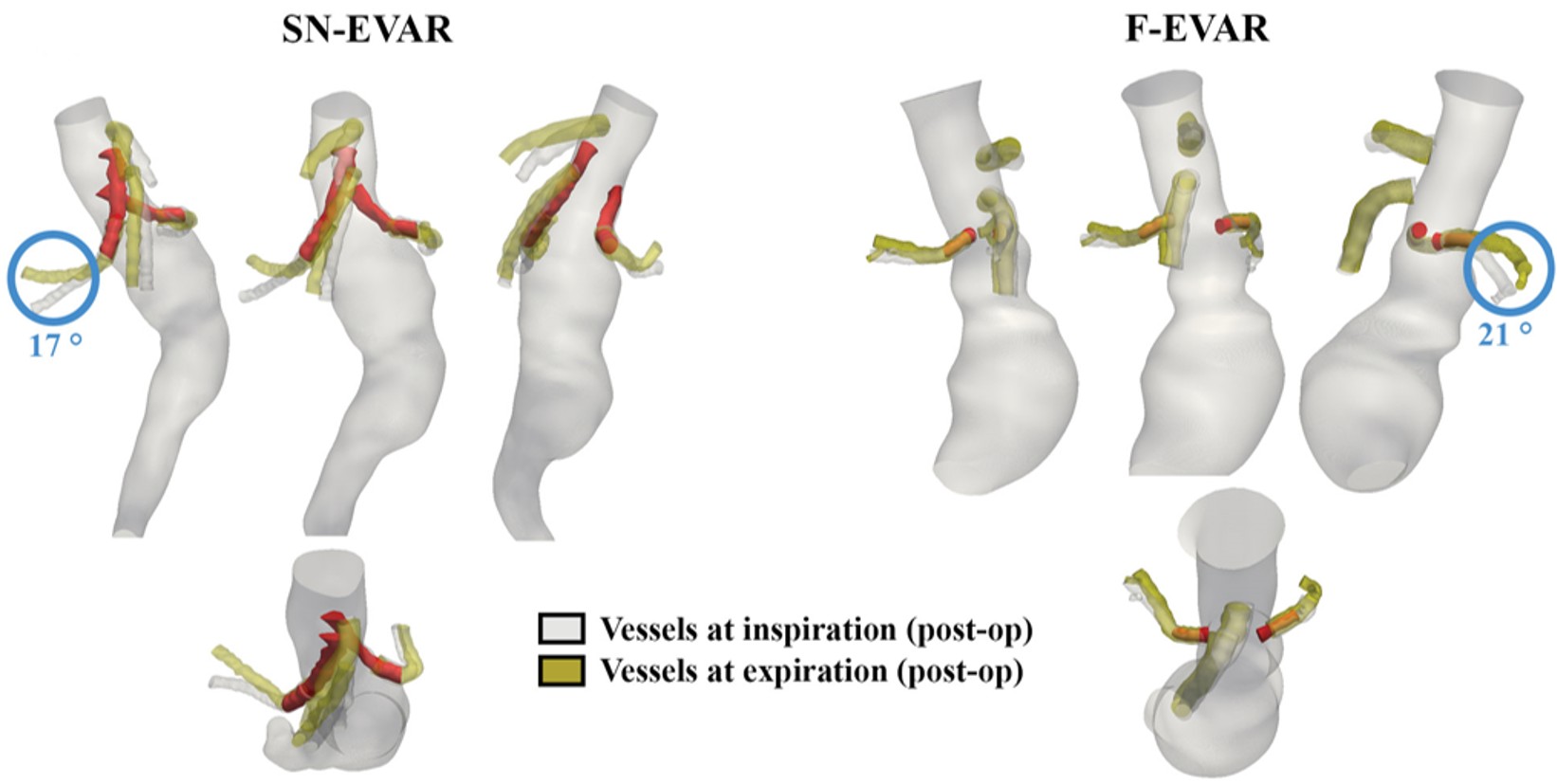
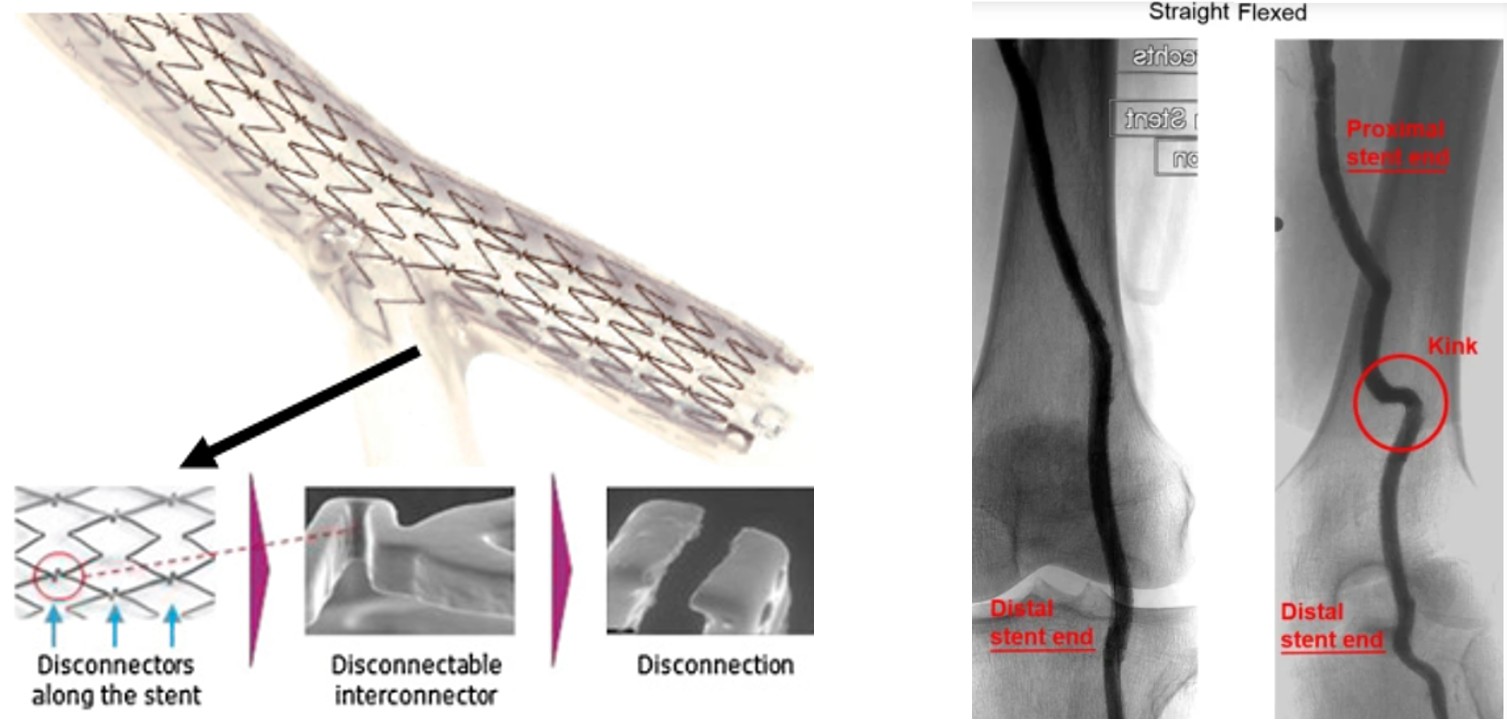
Grounding cardiovascular device development decisions in real biomechanical behavior
Durability issues, unexpected fatigue failures, and regulatory questions often originate from one root cause: unrealistic assumptions about the cardiovascular environment. Our new VIBE Solutions service addresses this gap by grounding device development decisions in real biomechanical behavior.
This service is supported by cooperation with Dr. Christopher Cheng—President of VIBE Solutions, Adjunct Professor of Vascular Surgery at Stanford University, President of CVID, and author of 100+ peer-reviewed publications including the Handbook of Vascular Motion.

Dr. Christopher Cheng
Our Mission
To improve the biomechanical compatibility of cardiovascular medical devices by applying deep expertise in cardiac and vascular motion, hemodynamics, medical imaging, device design, clinical practice, and regulatory strategy, supporting optimized device function and long-term durability.
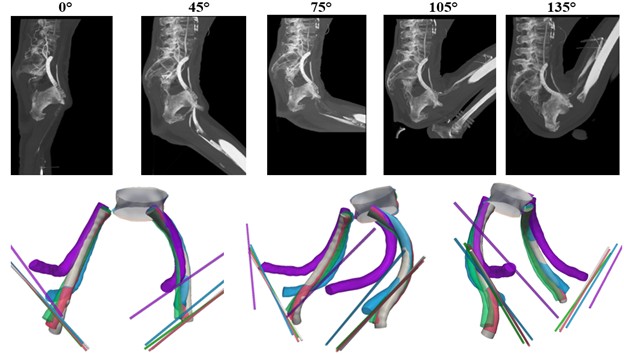
Scope of the Service
Comprehensive literature review and durability evaluation strategy.
- Cardiovascular biomechanics for device design, testing, and marketing messaging
- Preclinical, cadaver, and clinical imaging studies to acquire boundary conditions
- Execution of FEA and benchtop testing or working with your internal resources
- Nitinol processing for optimizing fatigue performance
- Partnering during the entire submission process until approval (FDA, CE, PMDA, etc.)
- Boundary condition and reduced durability testing rationale for regulatory submissions
- Mock pre-sub review by experts and former FDA reviewers
- Expert services for FDA Panels, litigation, root cause, etc.
- Clinical feedback from interventionalists (surgeons, radiologists, cardiologists)
- Biomechanical rationale for reducing clinical trial constraints, e.g., widening criteria to improve recruitment or narrowing criteria to reduce negative outcome risk
Door-to-door professor: Seasoned teacher to uplevel your team’s biomechanics knowledge
Testimonials
Rapid, Reliable Stent Study for Medtronic
“When we needed to derive deformation boundary conditions for stents in the tibial arteries, we knew exactly who to call. The study was executed smoothly and faster than agreed.”
Claudio Silvestro, R&D Program Director, Medtronic
Clinical Trial Acceleration for Rox
“Chris was able to widen our clinical trial ankle-brachial index inclusion criteria with a brilliant explanation that incorporated disease severity limits, hemodynamics, improved medical imaging protocol, and inclusion of diverse patient demographics. This saved us an immeasurable amount of time and money to complete our clinical trial.”
Rodney Brenneman, CEO, Rox Medical (then E3 MedTech)
Three FDA PMAs Achieved for Terumo
“Chris and his team performed a complex study to define the dynamic biomechanical environment of our thoracic aortic endograft and helped us earn three PMA indications from the FDA.”
ReadScott Rush, Global VP R&D, Terumo Aorticmore
Qualifications & Expertise
Experience
Over 50 years of combined experience across industry and academia, covering research, design, manufacturing, preclinical testing, clinical trials, and regulatory strategy
Certification
Biomechanical testing and analysis performed in ISO 13485–certified laboratories
Expertise
Led by experts with extensive academic, clinical, and industrial background in vascular biomechanics
References
Contribution to 100+ peer-reviewed publications and authoritative references in vascular motion and implant durability
Regulatory
Direct experience supporting FDA, CE, and PMDA submissions for cardiovascular devices
Contact us
Interested in our services? Send us a message and we will get back to you shortly.
Connect with us
Quick Links
By submitting this form, I acknowledge that I have read and understood the Privacy Policy and agree to the processing of my personal data for the purposes stated therein.